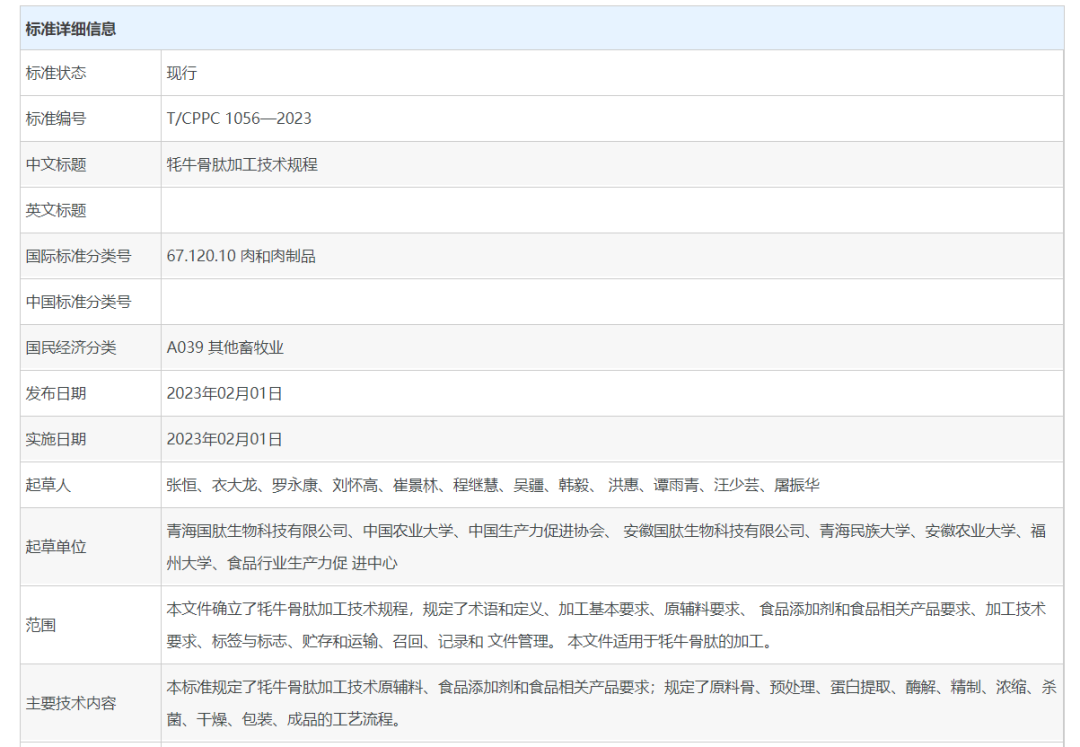
 2023-02-10
2023-02-10
First-class enterprises set standards, second-class enterprises focus on creating a brand, while third-class enterprises underline production. On February 1, 2023, the group standard Technical Specification for Processing Yak Bone Peptide, which was jointly prepared by industry-university-research institutes such as China Association of Productivity Promotion Centers, Productivity Promotion Center of Food Industry, China Agricultural University, Qinghai Minzu University, Anhui Agricultural University and Fuzhou University under the leadership of Qinghai Guotai Biotechnology Co., Ltd. and Anhui Guotai Biotechnology Co., Ltd., the subordinates of People’s Guotai Group, was released and implemented formally, which provides critical theoretical support and basis for the industrialization development of China’s yak bone peptide, and leads the standard construction of bioactive peptide industry continuously.

The standard sets down the processing procedures of yak bone peptide, such as basic conditions of production, technical requirements of processing and technological processes, in order to provide certain basis for production, ensure enterprises’ standard production, improve the quality & safety and standard level of yak bone peptide, promote the scientific, standard, systematic and industrial processing of yak bone peptide and enhance the competitiveness of China’s yak bone peptide products on the international market; it also brings certain significance to promote the sustainable development of comprehensive utilization industry of China’s yak bone byproducts.
As the enterprise representative for drafting this standard, Guotai has participated in formulating this group standard in the entire process in partnership with many authorities, and provided plentiful specific data support for the successful formulation of standard based on its long-term practice and experience in yak bone peptide industry. This standard specifies not only the requirements of processing technique, raw and auxiliary materials, food additives and food-related products of yak bone peptide, but also the full-cycle technological processing of raw material bone, pretreatment, protein extraction, enzymolysis, refining, concentration, sterilization, drying, packaging and finished products, in order to provide basis for enterprises’ standard production.

In the whole process of preparing group standards, the drafting team has strictly followed the requirements of relevant policies such as Food Safety Law, underlined food safety essentially, complied with the “normative, scientific, practical and advanced” principle and laid special emphasis on the universality, applicability and compatibility of standards. Formulate the specific implementation contents based on the standard, normalize relevant techniques for raw materials, processing, storage and transport of yak bone peptide by means of standard summary, enterprise research, analysis & verification, etc. and form normative exposure drafts and preparation instructions.

As a pioneer in China’s bioactive peptide industry, Guotai has insisted on the driving effect of technological innovation for over 10 years, and promoted the industry-university-research integrated development. It has also established the “one-institute two-station” (functional peptide research institute, academician workstations and professor workstations) together with authorities, to accelerate the transformation of scientific & technological innovation achievements into industrial achievements. At present Guotai has obtained over 120 patents. It endeavors to create a highland of scientific research and innovation industry, and constantly improves the discourse power in the field of bioactive peptide.

Qinghai Guotai Production Base will put into operation soon
By adhering to the strategy of “full industry chain development”, People’s Guotai Group has established three bioactive peptide production bases respectively in Langxi (Anhui Province), Changde (Hunan Province) and Xining (Qinghai Province) in succession, of which, the Qinghai Guotai invested with hundreds of millions RMB and focusing on the production and R&D of yak bone peptide will put into operation this year. Guotai’s three production bases will be put into operation jointly and work together to forge the largest bioactive peptide industry cluster in Asia. The annual yield may reach RMB 10 billion after the bases reach the design capacity, to constantly lead the industry’s high-quality and innovative development.

Presently, China’s bioactive peptide industry is undergoing transformation from “high-speed growth” to “high-quality growth”, but the industrial development is restrained by the problems such as incomplete industry standards and non-standard enterprise development. Therefore, standard construction is imperative, in order to promote industrial improvement and normalize enterprise development. In the future, Guotai will actively participate in the formulation of national, industrial and group standards based on its strengths in the whole industry chain, to gradually form the capability of outputting standards, and accelerate the standard development of the industry.
Company name: People's Guotai Group Co., Ltd
City: Beijing
Contact Person: Mr.Yang
E-Mail: 395655776@qq.com
Website: http://www.cc-gt.com/
Tel.: 400 009 1187